Hydrogen for transport and the B&E reportOil provides most of the energy for transportation and oil will soon be in short supply. That is the reason why advocates of the hydrogen economy want to replace oil with hydrogen. Practical hydrogen powered trucks and automobiles must be the initial objective. Hydrogen sources and a hydrogen distribution system will be needed. Much of what follows is a summary of a report, The Future of the Hydrogen Economy: Bright or Bleak? by two Swiss engineers, Ulf Bossel and Baldur Eliasson (B&E). [2] This report discusses hydrogen sources, hydrogen storage, and hydrogen distribution. Hydrogen powered vehicles are not discussed. This report was written by and for engineers. All assertions are supported by facts, figures and calculations. It is trustworthy in every way although it's a slog to read it. How do you get hydrogen?
|
| weight of fuel | weight of steel tank | weight of carbon fiber tank | volume of tank contents | volume of tank | |
|---|---|---|---|---|---|
| typical 18 wheel truck (diesel) | 1175 lb | (small) | NA | 22.5 feet3 | 24.0 feet3 |
| typical sedan (gasoline) | 108 lb | (small) | NA | 2.25 feet3 | 2.5 feet3 |
| truck converted to ICE hydrogen | 313 lb | 31,300 lb | 6,960 lb | 67.5 feet3 | 157 feet3 |
| sedan converted to hydrogen fuel cell | 17.4 lb | 1740 lb | 387 lb | 4 feet3 | 9 feet3 |
The space, weight and expense of steel tanks make them impractical. Any gains in energy efficiency would be offset by losses incurred in hauling the very heavy tanks. Carbon fiber tanks of this size and performance do not exist--they are only goals. Gasoline, by contrast, requires only a small, low-tech tank.
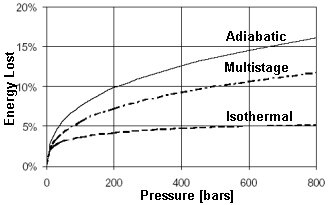 The
laws of thermodynamics dictate the amount of energy it takes to compress a
gas. The physical properties of hydrogen make it the most difficult of all
gasses to compress. At 800 bars, a perfect, single stage compressor
consumes energy equal to 16% of the chemical energy in the hydrogen. (This
is the energy that gets instantly released in the event of a tank
failure.) It is possible to use a multistage compressors with intercoolers
to achieve 12%. This is an estimate extrapolated from an actual multistage
compressor working at 200 bars. A multistage compressor working at 800
bars does not exist. [7]
The
laws of thermodynamics dictate the amount of energy it takes to compress a
gas. The physical properties of hydrogen make it the most difficult of all
gasses to compress. At 800 bars, a perfect, single stage compressor
consumes energy equal to 16% of the chemical energy in the hydrogen. (This
is the energy that gets instantly released in the event of a tank
failure.) It is possible to use a multistage compressors with intercoolers
to achieve 12%. This is an estimate extrapolated from an actual multistage
compressor working at 200 bars. A multistage compressor working at 800
bars does not exist. [7]
It is technologically challenging to compress hydrogen to 800 bars. Higher pressure would not result in much volume reduction. At these pressures, hydrogen acts less like a gas and more like a liquid.
The laws of thermodynamics also dictate that energy losses occur when hydrogen is transferred from a storage tank to a vehicle. The design of the transfer lines and the pressure fittings is critical in keeping energy losses low.
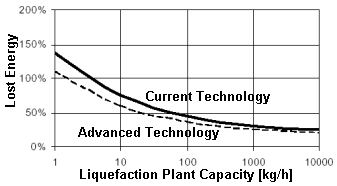 Liquid
(cryogenic) hydrogen also occupies 3 times more volume than gasoline for
the same energy. (Paradoxically, there is more hydrogen in a gallon of
gasoline than there is in a gallon of liquid hydrogen.) The advantage of
hydrogen liquefaction is that a cryogenic hydrogen tank is much lighter.
Hydrogen's physical properties means hydrogen is harder to liquefy than
any other gas except helium. There are significant and inevitable energy
losses when hydrogen is liquefied. This graph illustrates how energy
losses depend strongly on plant capacity. Losses
are 30% in the best case.
Liquid
(cryogenic) hydrogen also occupies 3 times more volume than gasoline for
the same energy. (Paradoxically, there is more hydrogen in a gallon of
gasoline than there is in a gallon of liquid hydrogen.) The advantage of
hydrogen liquefaction is that a cryogenic hydrogen tank is much lighter.
Hydrogen's physical properties means hydrogen is harder to liquefy than
any other gas except helium. There are significant and inevitable energy
losses when hydrogen is liquefied. This graph illustrates how energy
losses depend strongly on plant capacity. Losses
are 30% in the best case.
Liquid hydrogen is colder than any other substance except liquid helium. The advantage of liquid hydrogen is that it can be stored in relatively lightweight tanks. A tank for cryogenic hydrogen is like a thermos bottle, but it must work much better. It consists of a tank within a tank with a vacuum between the two. The inner tank must be supported without conducting heat from it. This is very difficult to do in a tank designed for a vehicle. Gasoline, by contrast, requires only a small, low-tech tank.
B&E estimates that a liquid hydrogen tank designed for automobile use will loose about 5% of its capacity every day, which is to say that all of it will be gone in 20 days. Losses of this magnitude are acceptable for, say, a taxicab fleet, but unacceptable to most people.
Hydrogen cannot be vented to the atmosphere because it is an explosion hazard and because it is a greenhouse gas. The vented hydrogen must be burned. A continuously running gas stove with one burner set to "medium" would do it.
Hydrogen molecules exist in two forms, ortho (the electron spins are anti-parallel) and para (the electron spins are parallel.) Room temperature hydrogen is a mixture of the two. However, liquid hydrogen turns into pure para hydrogen over the course of a few days. The process releases enough heat to turn the liquid hydrogen to gas in a few days. Liquid hydrogen can be catalytically converted to all para during the liquefaction process. If this were not done, 30% of the hydrogen would escape in two days even in a perfect cryogenic tank. Conversion adds to the cost and complexity of liquefaction.
Certain alkali metal hydrides release hydrogen when exposed to water. These metal hydrides hold enough hydrogen to make them useful for transportation. However, 70% of the energy is lost in the creation of the hydrides, making them unacceptable for widespread use.
Certain metals (platinum, zirconium, lanthanum) can be formed into "sponges" for hydrogen. But the "sponges" can hold only 1% of their weight in hydrogen and they are very expensive.
In both cases, the energy storage tank is heavy and expensive not to mention energy inefficient. These are not the qualities needed for vehicles designed for mass transportation. But the technology might have a niche application. Long lasting, but expensive, batteries for laptop computers may use these hydrogen sources to power small fuel cells.
It is possible to combine hydrogen with carbon to produce methane or gasoline, both superior carriers of energy. If the carbon is taken from the atmosphere there would be no net greenhouse gas contribution. The carbon would only be "borrowed" to carry the hydrogen. The gasoline made in this way would not contain carcinogens (like benzene) and it could be very high octane.
A 40 ton truck can deliver 26 tons of gasoline to a conventional gasoline filling station. One daily delivery is sufficient for busy station. A 40 ton truck carrying compressed hydrogen can deliver only 400 kilograms. That is because of the weight of the tank capable of holding 200 atmospheres of pressure. An empty truck will weigh almost as much as a full one. The compressed hydrogen tank must be robust. The energy used to compress the hydrogen to 200 atmospheres would be released instantly if a tank ruptured. The fireball would cover a football field. Hydrogen is more energy dense than gasoline (by weight) and hydrogen powered transportation is more energy efficient. Yet the hydrogen filling station will require 15 deliveries every day, everything else being equal. The energy cost of truck transport becomes unacceptable unless the source of hydrogen is very close to the point of use. A cryogenic truck could carry more hydrogen but recall that the energy cost to liquefy hydrogen makes this infeasible in most cases.
Hydrogen can be transported by pipeline. According to B&E, it take about 4 times more energy to move hydrogen through a pipeline compared to natural gas.
According to B&E, the hydrogen economy idea does not work for multiple reasons. They point out that there is no practical source of hydrogen, no good way to store hydrogen, and no good way to distribute hydrogen. Many of the problems of hydrogen stem from the physical and chemical properties of hydrogen. Technology cannot change these facts.
A compact and convenient energy carrier will be needed in the future. B&E suggest methane, ethane, methanol, ethanol, butane, octane, ammonia, etc. as better energy carriers.
It is difficult to understand the enthusiasm for hydrogen in view of the above. Hydrogen does not solve the energy problem and it is a bad choice for carrying energy.
![]()
 [1] http://www.bp.com/ British
Petroleum (BP) is a major oil company that publishes oil reserves data
from the Oil and Gas Journal in its Statistical Review of
World Energy. It is released annually in late June. The Review is widely referenced because it
is convenient, complete, colorful and very well done. Information about
hydro electricity, coal, and nuclear power are also included. It is also
big at 2.2 megabytes.
[1] http://www.bp.com/ British
Petroleum (BP) is a major oil company that publishes oil reserves data
from the Oil and Gas Journal in its Statistical Review of
World Energy. It is released annually in late June. The Review is widely referenced because it
is convenient, complete, colorful and very well done. Information about
hydro electricity, coal, and nuclear power are also included. It is also
big at 2.2 megabytes.
[2] The Future of the Hydrogen Economy: Bright or Bleak? This is the
April 2003, version 15 of the Bossel and Eliasson report. A 240 kilobyte
PDF file which may be viewed on this website by clicking here.
[3] The Oil Factor subtitled, Protect Yourself--and Profit--from the Coming Energy
Crisis, by
Stephen Leeb. A new (2004) book about investing and the energy
crisis. Leeb points out that the best use of wind or solar electricity to
free up oil, gas and coal to make transportation fuels (page 85). Leeb
also has a website:
http://www.leeb.net/
[4] http://www.princeton.edu/~hos/mike/texts/readmach/zmaczynski.htm Find out how the Haber process changed history.
http://www.llnl.gov/str/June03/Aceves.html Lawrence--Livermore National Laboratory tackles the problem of hydrogen storage for automotive use.
[5] http://www.eere.energy.gov/hydrogenandfuelcells/pdfs/h2_storage_think_tank.pdf
[7] http://www.tinaja.com/h2gas01.asp Don Lancaster's collection of hydrogen resources. Also called Don Lancaster's Guru's Lair. Don holds forth on a wide range of topics. Surprisingly, this is a good place to learn about and thermodynamics. The terms "adiabatic" and "isothermal" are explained. Don is a little bombastic but everything he says is backed up by facts.
http://mb-soft.com/public2/hydrogen.html A good website devoted to the idea of the Hydrogen Economy.
http://www.tipmagazine.com/tip/INPHFA/vol-10/iss-1/p20.html The January/February of 2004 issue of The Industrial Physicist has a feature article called Bottling the hydrogen genie.
http://www.hydrogenprodevel.com/ "Hydrogen Prodev Daily News" is hosted by a Phd Chemical Engineer. If you want detailed information about the hydrogen economy technology, this is a good place to look.